SPRINGDALE, Ark., Dec. 7, 2020 /PRNewswire/ — NOWDiagnostics, Inc. announced today that study results for its ADEXUSDx® COVID-19 antibody test yielded encouraging test performance—providing a strong use case for the innovative diagnostic device, which was filed for Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration (FDA) on May 29, 2020. The antibody test delivered 100 percent sensitivity and 100 percent specificity in a well-characterized 45 sample panel of COVID-19 positive and negative patient samples, provided by the Biodefense and Emerging Infections Research Resources Repository (BEI Resources) panel.

In August 2020, the Biomedical Advanced Research and Development Authority (BARDA), part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health and Human Services, provided funding and technical support to NOWDiagnostics for the delivery of a high-quality serological test for SARS-CoV-2 antibodies. The test can be used across a variety of health care settings—from hospital emergency rooms to clinics—and ultimately, by consumers for at-home use. As part of this cost-sharing contract, BARDA recommended evaluation of the ADEXUSDx® COVID-19 antibody test with the BEI panel.
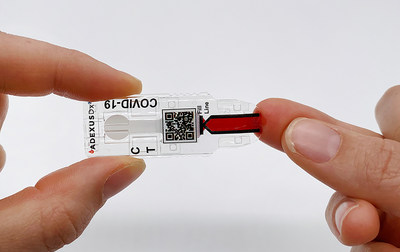
The ADEXUSDx® COVID-19 Test is a rapid serology, self-contained assay that measures the presence of SARS-CoV-2 antibodies and delivers lab-quality results in 15 minutes with no buffers, reagents, or additional equipment. The test requires only a drop of blood (either capillary blood from a simple fingerstick or 40 μL of venous whole blood, serum, or plasma) and has the potential to be deployed directly into homes and workplaces.
Reliable antibody tests will play a critical role in understanding the number of people who have been infected with COVID-19 and are potentially immune, as well as in the development and administration of COVID-19 therapies and vaccines. Availability of point-of-care (POC) and over-the-counter (OTC) tests would allow individuals to be screened easily and quickly, yielding test results in minutes instead of days.
NOWDiagnostics, Inc., based in Springdale, Arkansas, is a leader in innovative diagnostics testing. Its ADEXUSDx® product line features a lab at your fingertip, using a single drop of blood to test for a variety of common conditions, illnesses, and diseases, with results in a matter of minutes. By eliminating the need to send tests to off-site laboratories, NOWDiagnostics products have the potential to decrease the waiting period to determine test results by days. For more information about NOWDiagnostics, visit www.nowdx.com. For more information about the ADEXUSDx® COVID-19 Test, including its intended use, features, benefits and limitations, and directions for use, visit www.c19development.com. The ADEXUSDx® COVID-19 Test will be distributed by C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics. Laboratories may contact www.c19development.com/order to place an order.
Interested in learning more? You can read the full article here.
NOWDiagnostics, Inc., based in Springdale, Arkansas, is a leader in innovative diagnostics testing. Its ADEXUSDx® product line features a lab at your fingertip, using a single drop of blood to test for a variety of common conditions, illnesses, and diseases, with results in a matter of minutes. By eliminating the need to send tests to off-site laboratories, NOWDiagnostics products have the potential to decrease the waiting period to determine test results by days. For more information about NOWDiagnostics, visit www.nowdx.com. For more information about the ADEXUSDx® COVID-19 Test, including its intended use, features, benefits and limitations, and directions for use, visit www.c19development.com. The ADEXUSDx® COVID-19 Test will be distributed by C19 Development, LLC, a wholly owned subsidiary of NOWDiagnostics. Laboratories may contact www.c19development.com/order to place an order.
Contacts
NOWDiagnostics Company Contact
Beth Cobb, Director of Operations
Email: [email protected]
NOWDiagnostics Media Contact
Claire Burghoff, CGA Group
Email: [email protected]
