Monday, August 31, 2020
SPRINGDALE, AR, August 31, 2020 – NOWDiagnostics, Inc. announced today it is working with BARDA, the Biomedical Advanced Research and Development Authority arm of the U.S. Department of Health and Human Services, to develop a serological test for SARS-CoV-2 antibodies that can be used across a variety of health care settings—from clinics to hospital emergency rooms—and ultimately by consumers for at-home use.
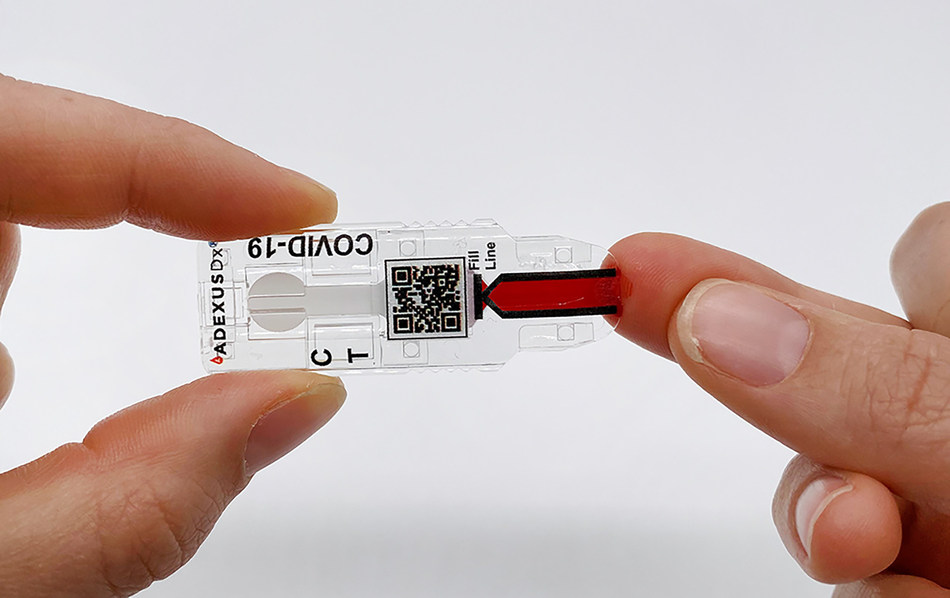
The COVID-19 rapid antibody test, using NOWDiagnostics’ self-contained ADEXUSDx® platform, requires no additional materials (reagents and buffers), equipment, processing, or refrigeration. No matter where it is conducted, the ADEXUSDx® COVID-19 Test is designed to deliver lab-quality results in 15 minutes detecting the presence of SARS-CoV-2 antibodies in individuals who have been exposed to the virus. This can include those who have been recently or previously infected with COVID-19, regardless of whether they presented with severe, moderate, mild or no symptoms.
The rapid-results ADEXUSDx® COVID-19 Test requires only a fingerstick to obtain 40 μL of capillary blood, venous whole blood, serum, or plasma and has the potential to be deployed directly in homes and workplaces. Availability of point-of-care (POC) and over-the-counter (OTC) tests would allow individuals to be screened easily and quickly, yielding test results in minutes instead of days. An application for Emergency Use Authorization (EUA) was filed with the Food and Drug Administration (FDA) in May 2020 for moderate complexity use of the ADEXUSDx® COVID-19 Test.
This project has been funded in part with Federal funds from the Department of Health and Human Services; Office of the Assistant Secretary for Preparedness and Response; Biomedical Advanced Research and Development Authority, Division of Research Innovation and Ventures under Contract No. 75A50120C00156. BARDA’s support for this project includes funding of $695,500 of the project costs and technical support required for NOWDiagnostics to request Emergency Use Authorization from the U.S. Food and Drug Administration (FDA) for POC and OTC uses of the ADEXUSDx® COVID-19 Test.
The information contained in this press release is provided by NOWDiagnostics, Inc. and does not indicate endorsement by the federal government of the company or its products.
NOWDiagnostics, Inc., based in Springdale, Arkansas, is a leader in innovative diagnostics testing. Its proprietary ADEXUSDx® platform features a lab at your fingertip, using a single drop of blood to test for a variety of common conditions, illnesses, and diseases, with results in a matter of minutes. By eliminating the need to send tests to off-site laboratories, NOWDiagnostics products have the potential to decrease the waiting period to determine test results from days to minutes. For more information about NOWDiagnostics, visit www.nowdx.com.
NOWDiagnostics Company Contact
Beth Cobb, Director of Operations
Email: [email protected]
NOWDiagnostics Media Contact
Claire Burghoff, CGA Group
Email: [email protected]